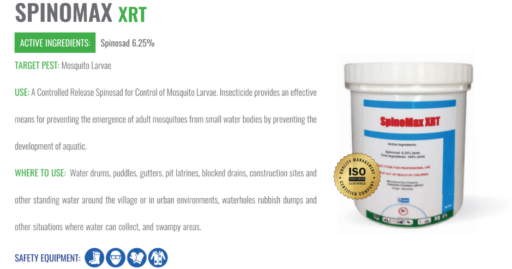
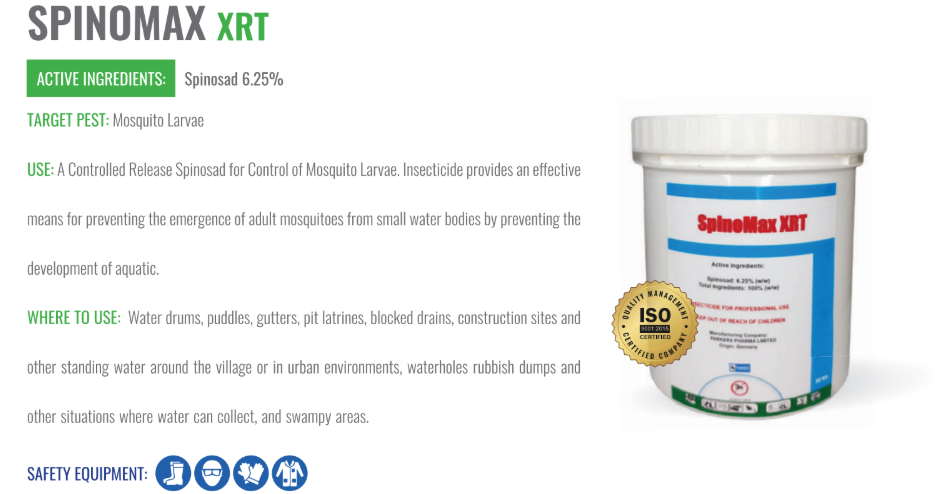
Parkers Pharma has launched Spinomax XRT, a slow-release larvicide tablet designed to last 180 days, offering sustained mosquito control for regions vulnerable to vector-borne diseases such as malaria, dengue, chikungunya, Zika virus, and Rift Valley fever.
Developed under the direction of Dr Wathek Zair, Spinomax XRT marks a pivotal advancement in larval source control—particularly in areas where frequent treatment is impractical due to resource or logistical limitations.
Scientific Mechanism and Targeted Action
The formulation contains 6.25% Spinosad, a naturally occurring compound extracted from Saccharopolyspora spinosa. This agent works by targeting mosquito larvae’s nervous systems, disrupting nicotinic acetylcholine receptors, which leads to paralysis and rapid mortality. The extended-release design ensures a consistent release of Spinosad for up to six months in breeding habitats.
Versatile Usage and Proven Impact
Spinomax XRT is adaptable for deployment across a wide range of environments, including:
-
Domestic water storage containers
-
Agricultural water collection points
-
Urban construction zones
-
Wetlands and seasonal floodplains
-
Humanitarian camps and informal settlements
Trials indicate larvicidal activity begins within 4 to 24 hours of use. The product has demonstrated high efficacy against key mosquito species—Anopheles, Aedes, and Culex—which transmit the world’s most dangerous diseases. To date, resistance to Spinosad has not been observed in these vector populations.
Expert Commentary
“Spinomax XRT is one of the most promising larvicides we’ve tested. Its long-term release and rapid action allow us to prevent mosquito emergence in areas where daily treatment isn’t feasible. This is a major step forward in vector control.” — Dr Emma Caldwell, Senior Vector Biologist, Centre for Tropical Disease Control, UK
Global Authorisation and WHO Alignment
The product is currently under regulatory review across numerous endemic nations in Africa, Asia, and Latin America. Its composition aligns with WHO guidance for larval source management, especially in settings where adulticide resistance or access issues limit other control methods.
Advancing Global Health Solutions
“This development reflects our commitment to deliver practical, science-driven tools for global disease control,” said Dr Wathek Zair, scientist at Parkers Pharma. “By extending the duration of larvicide effectiveness, we’re addressing a critical gap in current vector control strategies.”